Biophysics Laboratory
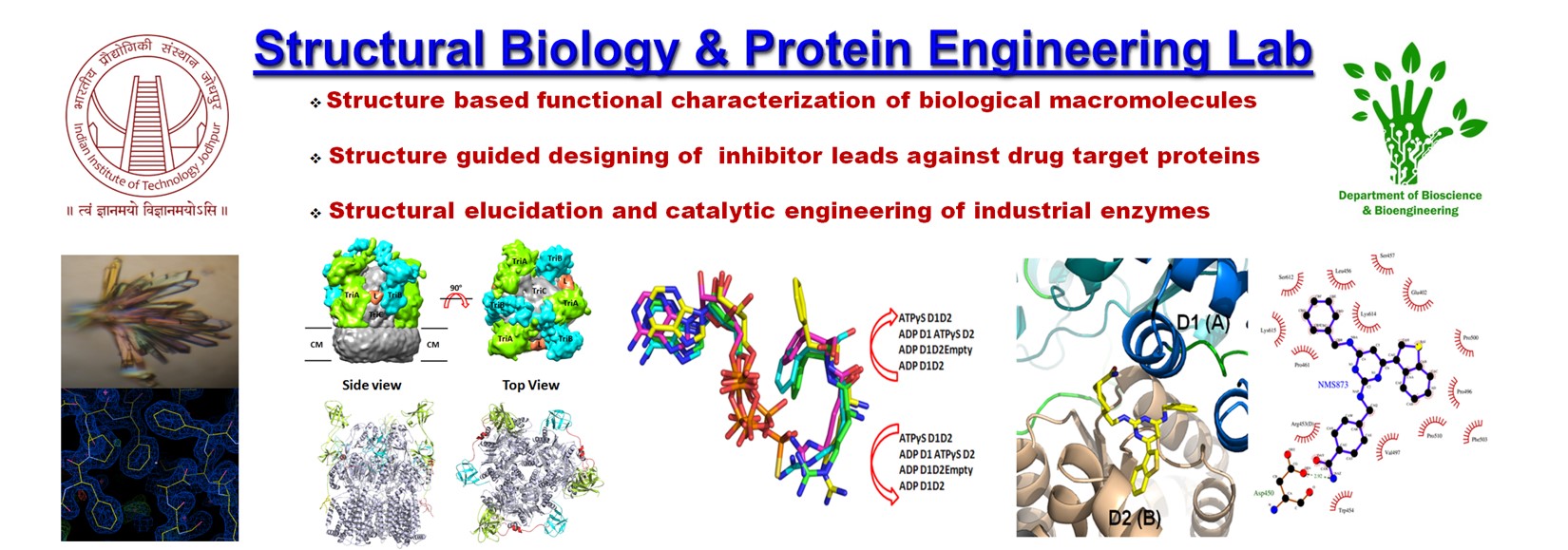
“Seeing is believing”…according to this famous quote, visual inspection paves the most convincing way to divulge naturally occurring phenomena. In Structural Biology and Protein Engineering lab, we aim to elucidate complex biological phenomena by unraveling the molecular snapshots of the concerned pathways through the atomic resolution structures of the macromolecules involved. For this purpose, we principally use the cutting edge tools of structural biology (X-ray diffraction crystallography and single particle Cryo-Electron microscopy) to define the structure-function behavior of biological macromolecules.
Faculty Members associated with the lab

Sudipta Bhattacharyya
Assistant Professor
Neha Jain
Assistant ProfessorGroups under this theme
1. Structural Biology & Protein Engineering Group |
Our main focus is to deduce the molecular mechanism of diseased conditions. Likewise, taking a reductionist’s approach we target proteins or protein complexes involved in disease progression and pathogenesis to reveal their molecular mechanism of action. The detailed three dimensional structural information gained thereof, not only pinpoint the role of the target proteins/protein complexes but also help us to design structure based lead inhibitor libraries against these potential drug target candidates. Further kinetic characterization of these potential inhibitor leads against the drug candidate proteins is performed to validate their antagonistic properties in vitro. The structural biology and protein engineering lab also aims industrially important enzymes to customize their structure-function to cater industrial needs. The high resolution structural information of the industrially important protein(s)/enzymes(s) also enable us to gain significant insights into their structure-function relationships which in turn allow us to tailor their function by the state of the art protein engineering and bioinformatic tools.
|
2. Protein Aggregation & Amyloid Biology Group |
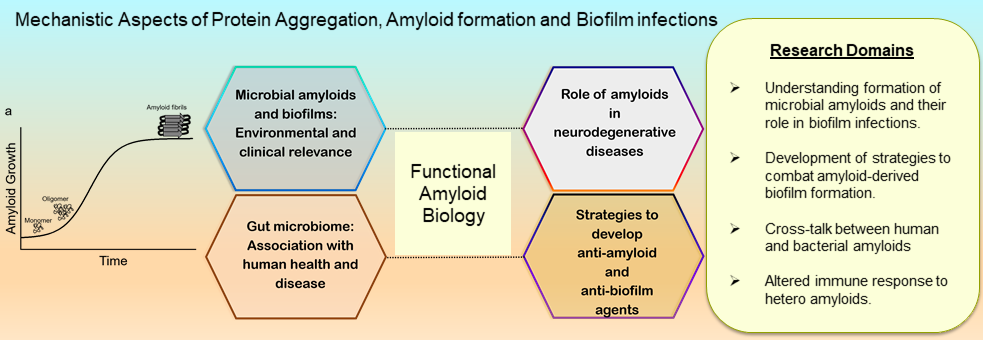
The lab is interested in understanding the altered folding of proteins which leads to formation of ordered aggregates called amyloids. Amyloids are highly stable ordered cross-β-sheet aggregates of proteins which are considered as the hallmark of neurodegenerative disorders like Parkinson’s and Alzheimer’s. We are interested in looking at amyloids that have both functional and disease-associated properties.
We utilize various biophysical, biochemical and microbiological tools to answer some of the fascinating questions pertaining to amyloids fibrils. In particular we are interested in understanding two aspects of human amyloids: 1. How bacterial amyloids influence the aggregation of human amyloids and contribute to progression of neurodegenerative diseases? 2. Development of unconventional strategies to modulate amyloid formation in humans. |
Projects that the lab is currently catering to |
1. Structure based functional characterization of Staphylococcal thiol peroxidase enzymes: A new hunch for anti-Staphylococcal drug discovery (Dr. Sudipta Bhattacharya) To explore a new avenue of anti-Staphylococcal drug designing research through structure based functional identification of thiol based antioxidant system |
2. Multimodal Approaches to Develop Potential Therapeutic LeadsTargeting Molecular Hot Spots of Duchenne Muscular Dystrophy for Clinical Trial (Dr. Sudipta Bhattacharya, Co-PI) (Approved) Molecular identification, characterization and validation of potential therapeutic agents for Duchhene Muscular Dystrophy |
3. Modulation of α-synuclein amyloid assembly using human chaperone like proteins (Dr. Neha Jain) Most of the human proteins rarely but remarkably undergo spontaneous conformational change in response to genetic or environmental stimuli. α-Synuclein is one such protein that undergoes conformational switch resulting in amyloid formation that is linked to Parkinson’s Disease (PD) progression. The present status of research on α-synuclein aggregation and PD progression reveals that the traditional therapy may not work to cure the disease in the long term. There is dire need of new therapeutic interventions that can either prevent PD or suppress it at the earlier stage of progression. The role of innate chaperone-like proteins in circumventing α-synuclein amyloid assembly and their potential as future drugs is still in its infancy. Here, we plan to explore the anti-amyloid activity of chaperone-like proteins against synuclein aggregation |